Bath time for blood cells: improving the red blood cell washing process
In brief...
Implementing a new washing system for red blood cells resulted in a blood product with longer shelf-life while maintaining patient safety and transfusion efficacy.
As the metabolites are the same ones normally produced in the body, they pose no risk to the majority of transfusion recipients. However, for some patients the metabolites can be harmful. For example, newborns are particularly sensitive to potassium, a metabolite that can affect heart function. In addition, certain metabolites and proteins can be harmful to patients who need regular blood transfusions or those who have a history of bad transfusion reactions. Other at-risk recipients are those who lack IgA-type antibodies. These patients could have severe reactions if they receive blood containing IgA. For all these patients, Canadian Blood Services or Canadian hospitals perform an additional processing step on the red blood cells: they are washed!
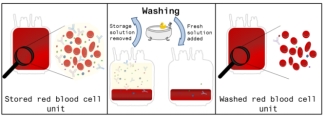
During washing, most of the metabolites and proteins that accumulated in the bag during storage are removed resulting in a safer product. Up until late 2013, Canadian Blood Services used an ‘open’ system to wash red blood cells. After washing with the ‘open’ system, the products are at greater risk of becoming contaminated with bacteria and must be used within 24 hours. This can cause issues in getting these special products to patients. In 2011, Canadian Blood Services began investigating a new process to wash the cells within a ‘closed’ system and store them in a solution containing nutrients as this allows the cells to be stored for longer after washing.
What did the researchers do?
Using standard methods to evaluate the quality characteristics of red blood cells and to measure levels of metabolites, the researchers tested the new ‘closed’ system (the ACP-215 from Haemonetics). They determined the best storage time before and after washing and the best nutrient solution. They also examined the quality of ‘washed and irradiated’ red blood cells. Irradiation is an additional step to prevent bad transfusion reactions and is recommended for some of the same patient groups that need washed red blood cells. After the new washing system was introduced in October 2013, the researchers monitored its impact on safety, efficacy and use of washed red blood cells by examining hospital data.
What did the researchers find?
- The quality characteristics of red blood cells washed with the new system were equal to, or better than, those washed using the old system, and were best maintained in a nutrient solution called saline-adenineglucose-mannitol (SAGM).
- Washing red blood cells within the first two weeks of storage and storing the washed red blood cells for a maximum of one week after washing was the best way to maintain an acceptable level of quality.
- Quality was best maintained in ‘washed and irradiated’ red blood cells when they were irradiated within a week of washing, and used within two days of irradiation.
- Under these conditions, both washed and ‘washed and irradiated’ red blood cell products met requirements for all uses except for transfusion to IgA-deficient recipients. Levels of IgA remaining after washing were too high to be safe for these recipients.
- Washing the red blood cells twice could reduce IgA levels to less than 0.05 mg/dL making them suitable for transfusion to IgA-deficient recipients.
- Once the new washing system was implemented adverse transfusion reaction rates did not change, occurring in 0.15 per cent of transfusions both before and after implementation of the new system.
- The longer storage time after washing reduced the number of red blood cell units that were discarded due to expiry, from approximately 4 per cent of all washed units before implementation to less than 1 per cent after implementation. Utilization of washed red blood cell units by the hospitals improved.
How can you use this research?
With the introduction of any new process within Canadian Blood Services, the primary goal is to maintain patient safety. These “bench to bedside” studies to investigate, implement and validate the new washing process took several years and allowed the implementation of a new system that not only ensures patient safety but also improves blood product utilization. The new washing system has clear advantages over the old. Most important of these is the longer storage time after washing (from 1 day to 1 week).
This advantage has reduced the number of washed red blood cells that are discarded before they are used: preventing product wastage, saving money and improving efficiency. It has made it easier for Canadian Blood Services to provide washed products to hospitals and for the hospitals to provide those washed products to the patients that need them. Since the new system was introduced, the use of washed red blood cells has increased at hospitals while safety and transfusion efficacy were maintained.
Interestingly, there was a small decrease in the number of units transfused per transfusion episode after implementation, reducing the exposure of recipients to blood products. Use of the new system to wash other products such as cryopreserved (frozen) red blood cells components is being investigated.
About the research team
This research was conducted in the laboratory of Dr. Jason Acker, a senior development scientist with Canadian Blood Services’ centre for innovation and a professor in the department of laboratory medicine and pathology at the University of Alberta in Edmonton. Based at Dr. Acker’s laboratory, Adele Hansen is project lead with Canadian Blood Services’ centre for innovation, and Tracy Turner and Jayme Kurach are research assistants. Dr. Qi-Long Yi is a senior biostatistician with Canadian Blood Services centre for innovation in Ottawa. Dr. Barbara Hannach is a Canadian Blood Services medical officer based in Toronto. Nayana Sondi is an applications specialist and Dr. Christine Cserti-Gazdewich and Dr. Jacob Pendergrast are physicians and transfusion medicine specialists at the University Health Network in Toronto.
This research unit is derived from the following publication(s)
[1] Hansen A, Yi Q, Acker JP: Quality of red blood cells washed using the ACP 215 cell processor: assessment of optimal pre- and postwash storage times and conditions. Transfusion 2013;53:1772-79.
[2] Hansen AL, Turner TR, Yi Q, Acker JP: Quality of red blood cells washed using an automated cell processor with and without irradiation. Transfusion 2014;54:1585-94.
[3] Hansen AL, Turner TR, Kurach JDR, Acker JP: Quality of red blood cells washed using a second wash sequence on an automated cell processor. Transfusion 2015;55:2415-21.
[4] Acker JP, Hansen AL, Yi Q, Sondi N, Cserti-Gazdewich C, Pendergrast J, Hannach B: Introduction of a Closed-System Cell Processor for Red Blood Cell Washing: Post-Implementation Monitoring of Safety and Efficacy. Transfusion 2016;doi: 10.1111/trf.13341.
Acknowledgements: This research was supported financially by Canadian Blood Services, funded by the federal government (Health Canada), provincial and territorial ministries of health. The views expressed herein do not necessarily represent the view of the federal government. Canadian Blood Services is grateful to blood donors for making this research possible.
Keywords: Red blood cell, washing, IgA, neonates, adverse reactions, process, metabolites, utilization, efficacy, safety
Want to know more? Contact Dr. Jason Acker at jason.acker@blood.ca.
Research unit is a knowledge mobilization tool developed by Canadian Blood Services’ Centre for Innovation